FAQ: Hospital tubing management questions
Who is using it?
Discover our map showcasing locations that have embraced The Beata Clasp medical line organizer, from hospitals to rehab centers, government facilities, and even veterinarian clinics!
Do patients like the Beata Clasp?
Yes! Patients appreciate being involved in their care, which improves both compliance and safety. The Beata Clasp helps keep their environment organized and reduces the risk of line entanglement. Check out our Testimonials to see what patients and healthcare professionals are saying!
Do you sell to other countries?
Yes, we do. We are already established in several countries, and our distributors have stock on hand for faster delivery and reduced shipping costs. Please refer to our International Distributor Page for ordering outside of the United States.
How can I be sure the Beata Clasp will fit my equipment, such as a bedrail?
The Beata Clasp is designed with flexibility to fit a wide range of bedrails and other equipment. We encourage facilities to request a sample to evaluate fit and functionality in their specific setting. For added securement, we offer an optional reusable yellow fastener tie for purchase.
What size tubing can the Beata Clasp accommodate?
Each hole in the Beata Clasp can accommodate up to 5/8 of an inch of tubing (0.625 inches / 15.875 mm). The chart below shows examples of standard medical tubing sizes that fit through the grooves:
The grooves on the top of the product are adjustable and flexible, allowing you to insert any tubing that fits within the maximum diameter of the hole.
Tip: Allow extra slack in the tubing if passive range of motion is needed.
Avoid placing very sensitive lines in the Clasp if the patient is not alert and oriented or is at risk for the tubing being tugged or pulled.
Each hole can hold multiple IV lines or smaller cords together if needed.
Is the Beata Clasp intended for single or multiple patient use?
Recommended Use: The Beata Clasp is recommended as a single-patient item that begins use in the emergency department or upon admission and remains with the patient throughout their stay. The product may go home with the patient at discharge.
Additionally, we offer a recycling program for facilities looking to dispose of used clasps in an environmentally friendly way.
Even as a single-patient-use product, The Beata Clasp supports environmentally conscious care by providing long-lasting use throughout a patient’s stay and beyond, reducing the need for repeated disposable workarounds or replacement equipment.
How do you clean the Beata Clasp?
The Beata Clasp can be cleaned an unlimited number of times using soap and water, bleach, or any standard sanitizing disinfectant wipes available at your facility. Its durable, non-porous material ensures easy cleaning without compromising its integrity. However, it cannot be autoclaved.
The CDC Isolation Guideline recommends that “noncritical equipment contaminated with blood, body fluids, secretions, or excretions be cleaned and disinfected after use.” Additionally, for certain pathogens that can survive on surfaces for prolonged periods, the guideline advises disinfection with an EPA-registered low or intermediate-level disinfectant. Consistent cleaning and disinfecting of bedside equipment help reduce infection risks, protect patient safety, and lower healthcare costs.
For more details on best practices, refer to the CDC Isolation Guideline.
How can I get my facility to use the Beata Clasp?
Many RNs and CNAs introduce the Beata Clasp to their manager or present it to a unit-based council or committee for review. Once approved, your facility’s purchasing department can contact us to place an order and stock your unit. To help with the process, download our product brochure and share it with your manager.
What education is there to support the use of a medical line organizer?
We provide multiple educational resources to support the use of the Beata Clasp medical line organizer in healthcare settings:
- Explainer & Training Video: Our newly created one-minute video serves as a quick in-service or training tool for clinical staff. It demonstrates how to attach the Beata Clasp to a bed rail and provides a clear overview of its benefits in preventing tubing mismanagement.
- Industry Insights on Patient Safety: We reference key findings from ECRI’s Top 10 Health Tech Hazards for 2025 report. The report highlights infection risks and tripping hazards from poorly managed infusion lines (#8 on the list)—a critical issue the Beata Clasp helps address.
- Webinar on Preventing Medical Line Entanglement: This in-depth session discusses the risks of tubing misconnections, medication errors, and patient falls due to mismanaged lines. The webinar highlights how the Beata Clasp promotes line tracing from the patient to the source, reducing the risk of errors.
- Compliance & Best Practices: We offer guidance on how hospitals can proactively answer The Joint Commission’s inquiries regarding medical line entanglement. The Beata Clasp helps facilities implement best practices for tubing management, keeping lines off the floor, preventing falls, and ensuring standardized patient safety protocols.
- Regulatory boards, compliance, and recommendations PDF
For more information, access our video resources, attend our educational webinars, or reach out to us directly. We’re happy to support your facility in improving patient safety and workflow efficiency.
Do you have a policy to prevent tubing misconnections?
Yes. View a sample Hospital Operational Procedure Manual on Prevention of Tubing Misconnections at this link.
Purpose: Ensure safe medical tubing practices.
Download Policy for full details.
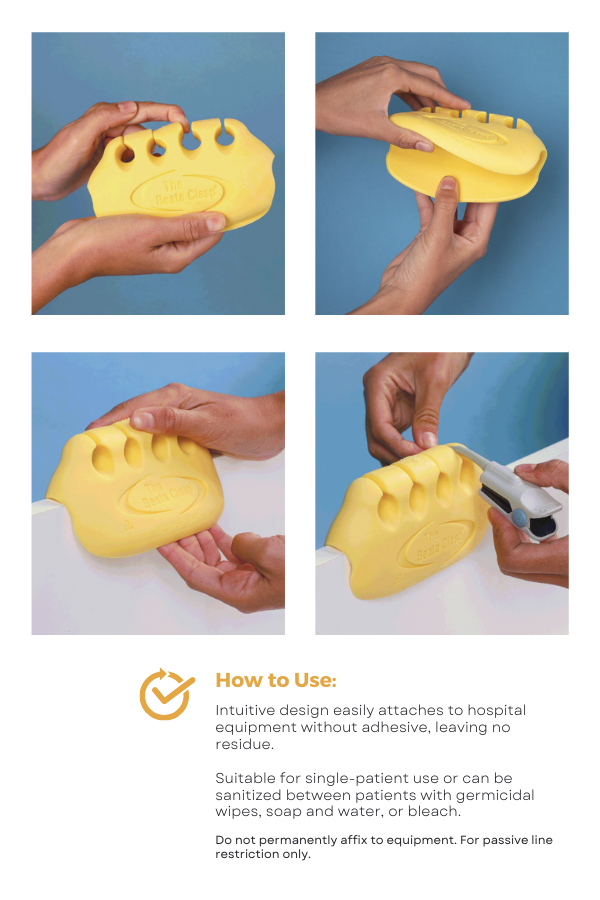
What are the suggested guidelines for use of the Beata Clasp?
The Beata Clasp® Line Organizer Guidelines : Downloadable PDF
Includes:
- Purpose of hospital tubing and line organization
- Compatible tubing and catheter types
- Step-by-step usage instructions
- Attachment and removal procedures
- Cleaning guidelines
Download now for detailed guidance on safe and effective use!
What is a workaround?
Workarounds circumvent or temporarily ‘fix’ perceived workflow hindrances to meet a goal or to achieve it more readily. Behaviours fitting the definition of workarounds often include violations, deviations, problem solving, improvisations, procedural failures and shortcuts.
Clinicians implement workarounds in response to the complexity of delivering patient care. One imperative to understand workarounds lies in their influence on patient safety. See Examples Here.
What is The Beata Clasp Made Of?
The Beata Clasp is made from a soft, flexible, rubber-like plastic known as a styrenic thermoplastic elastomer (TES), which provides durability while remaining lightweight. It is latex-free, making it safe for individuals with latex allergies. The material contains an antimicrobial additive, which helps prevent the growth of bacteria and mold, ensuring a cleaner and safer product for medical environments. While it can be stored at a range of temperatures, it cannot be autoclaved (not suitable for high-heat sterilization).
On a technical level, the Beata Clasp is composed of a styrenic-polyolefin thermoplastic rubber blend with a foaming agent.
Where can I find the MSDS for this product?
Click here to download the MSDS PDF for our yellow antimicrobial Beata Clasp: MSDS PDF
Do you sell to government facilities?
Yes, we are registered with the U.S. federal government's System for Award Management (SAM) under LENORE HENNING ENTERPRISES, INC. (NCEVU3VAVUL6 / 7HML5) and are authorized to sell to government facilities.
Is the Beata Clasp classified as a medical device, and does it require FDA registration?
The Beata Clasp is not classified as a medical device and does not require FDA registration. It falls under the category of noncritical reusable medical equipment, similar to bedpans, blood pressure cuffs, crutches, and computers. According to the CDC, noncritical items pose virtually no risk of transmitting infectious agents when used appropriately, as they do not come into contact with non-intact skin or mucous membranes.
What are people saying?
Visitors and patients worry about Line management at the bedside when done poorly. See threads for their perspective.
What certifications or awards has The Beata Clasp earned?
SupplierGATEWAY Diverse Supplier Certification - Woman Owned & Small Business (NAICS 423450
The Beata Clasp is a certified Woman-Owned and Small Business enterprise in the United States, verified through SupplierGATEWAY. We have also earned industry-recognized credentials in human factors, healthcare value analysis, and safety.
How do I ask an additional question?
We would love to hear from you! Please send your questions via our contact form or email us at contact@beataclasp.com. We can also be reached at 1 (800) 796-5840.
